Figure
Written by: Gunn Ochaphan
Contact: gunnochaphan@gmail.com
Cement comes from limestone. Limestone is a soft, light-colored rock with a grainy texture. The key ingredient in limestone is calcium. Limestone is a sedimentary rock typically formed from crushed seashells. (Chalk is also a form of limestone, calcium is basically “chalk-element”)

Firstly you crush the limestone into powder then you burn it to turn calcium carbonate (CaCO3) into calcium oxide (CaO). Calcium oxide is caustic powder, traditionally known as “quicklime” (“quick” means “active, alive”). When quicklime is combined with water. The water does cool it and it also reacts with the water and give off heat. The result is Ca(OH)2, Calcium hydroxide, aka “slaked” or “hydrated” lime
If you then pour slaked lime into a mold. It will absorb CO2 from the air and turn back to CaCO3 (and give off water vapor) In other words, when cement sets it turns back into limestone
Basically we crush and burn rock, and then re-constitute it at a time and place in a form we choose
Now a pure lime mixture like this isnt very useful. To use in construction, you need to mix it with something to give volume and strength
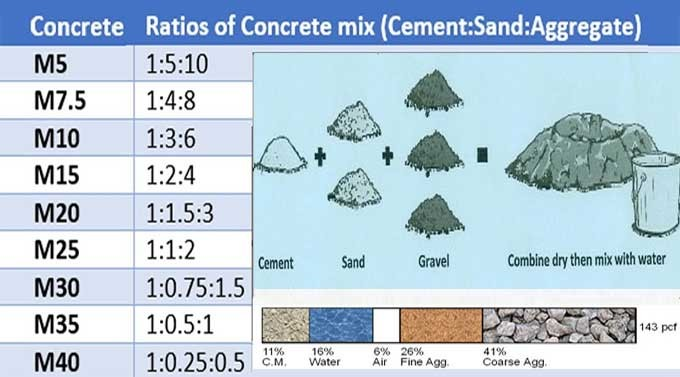
Those are basics, but there are many advanced formulas for cement One problem is that cement is strong under compression but not tensile
During industrial revolution, we figured out how to reinforce concrete with iron bars These reinforcing bars, or “rebar” give concrete tensile strength

next article, we'll learn more about modern technologies of cement.